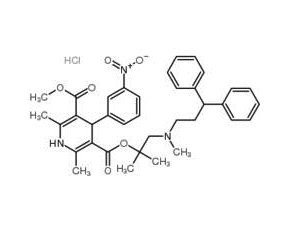
Lercanidipine hydrochloride
CAS No. 132866-11-6
Lercanidipine hydrochloride ( Lercanidipine hydrochloride;Renovia; Vasodip; R 75 )
Catalog No. M17950 CAS No. 132866-11-6
Lercanidipine is a calcium channel blocker of the dihydropyridine class.
Purity : 98%
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 10MG | 41 | In Stock |


|
| 25MG | 81 | In Stock |


|
| 50MG | 153 | In Stock |


|
| 100MG | 239 | In Stock |


|
| 200MG | 305 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameLercanidipine hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionLercanidipine is a calcium channel blocker of the dihydropyridine class.
-
DescriptionLercanidipine hydrochloride is a calcium channel blocker used in the treatment of hypertension.
-
SynonymsLercanidipine hydrochloride;Renovia; Vasodip; R 75
-
PathwayEndocrinology/Hormones
-
TargetAChR
-
RecptorCalcium Channel
-
Research AreaCardiovascular Disease
-
Indication——
Chemical Information
-
CAS Number132866-11-6
-
Formula Weight648.19
-
Molecular FormulaC36H41N3O6·HCl
-
Purity98%
-
SolubilityDMSO : 50 mg/mL 77.14 mM; H2O : < 0.1 mg/mL
-
SMILESCC1=C(C(C(=C(N1)C)C(=O)OC(C)(C)CN(C)CCC(c1ccccc1)c1ccccc1)c1cc(ccc1)[N+](=O)[O-])C(=O)OC.Cl
-
Chemical Name3-(1-((3,3-diphenylpropyl)(methyl)amino)-2-methylpropan-2-yl) 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate hydrochloride
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Gigant B, et al. Nature, 2005, 435(7041), 519-522.
molnova catalog


related products
-
TA-03
TA-03 is a potent inhibitor of AChE in treatment of cognitive manifestation of AD.
-
Dichlorisone Acetate
It belongs to organophosphate compounds, used as a kind of common environmental health insecticide.
-
Nicotine Ditartrate
(?)-Nicotine is the dominant form of the natural alkaloid nicotine. It acts as an agonist of neuronal nicotinic acetylcholine receptors (nAChRs) and possesses addictive and teratogenic properties. (?)-(S)-Nicotine is significantly more active at binding nAChRs compared to the (+)-(R) antipode thus nicotine is typically synthesized as (?)-(S)-nicotine with only 0.2-1% of the (+)-(R) isomer present.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


